| Sign In | Join Free | My frbiz.com |
|
| Sign In | Join Free | My frbiz.com |
|
| Categories | Antigen Rapid Test Kit |
|---|---|
| Brand Name: | LABNOVATION |
| Model Number: | LX-401301 |
| Certification: | ISO13485,ISO9001,CE |
| Place of Origin: | China |
| MOQ: | 10000 Pieces |
| Price: | Negotiation |
| Payment Terms: | T/T, D/A, D/P |
| Supply Ability: | 500000/Day |
| Delivery Time: | 5-7 work day |
| Packaging Details: | Carton |
| Quanlity Guarantee Period: | 24 months |
| Storage Condition: | 2℃-30℃ |
| Test Time: | Within 15 min |
| Group: | Universal |
| Sunshine: | Not Allowed |
| Material: | Cassette |
| Company Info. |
| Labnovation Technologies, Inc. |
| View Contact Details |
| Product List |
SARS-CoV-2 Antigen Rapid Test Kit Professional Diagnostic Vireus Antigen Use CE Certificated German BfArm List Approved
Intend Use

Product Details
| Item | Value |
| Model Number | LX-401301 |
| Type | 20 Tests/Kit |
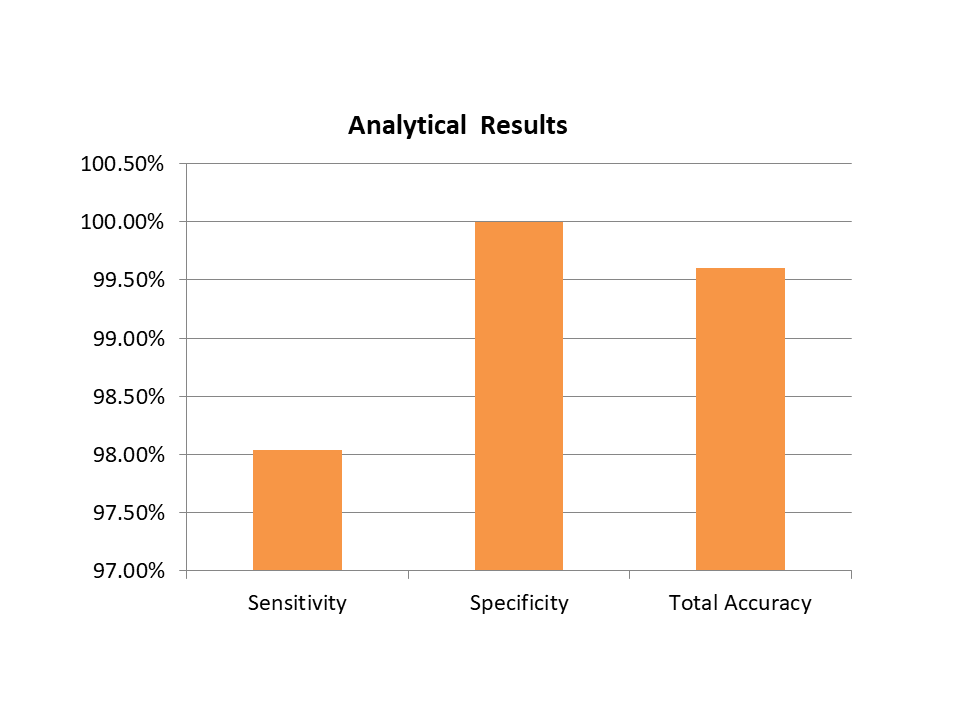
| Specificity | 100% |
| Sensitivity | 98.04% |
| Accuracy | 99.60% |
| Warranty | 24 Months |
| Quality Certification | CE,MSDS |
| Safty Standard | ISO13485 |
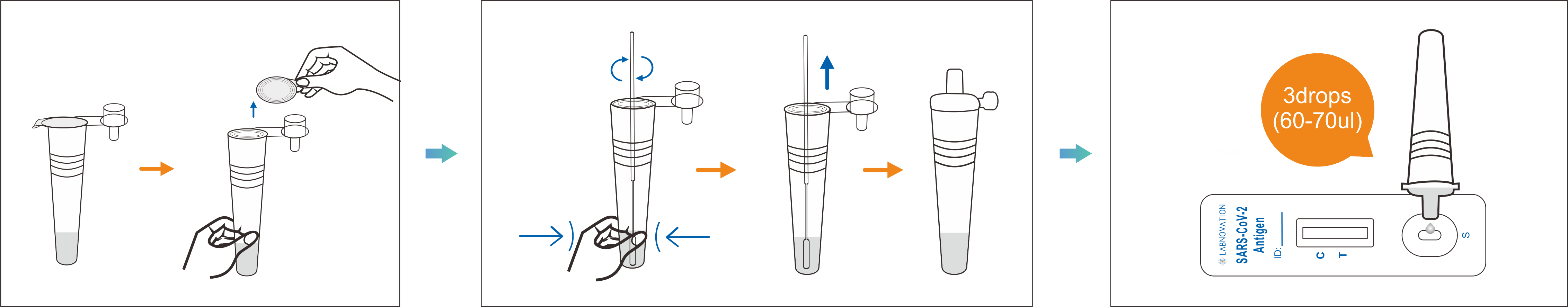
Sample Volum | 3 Drops |
Main Components
Analytical Results
Product Features
Sample Preservation
Sample of human nasopharyngeal swabs and oropharyngeal swabs should be processed as soon as possible after sample collection. If the test cannot be performed immediately, the sample should be stored in a sealed state, stored at 2~8℃ for 8 hours, and stored below -20℃ for 1 month. Long-term storage is not recommended.
Use Step
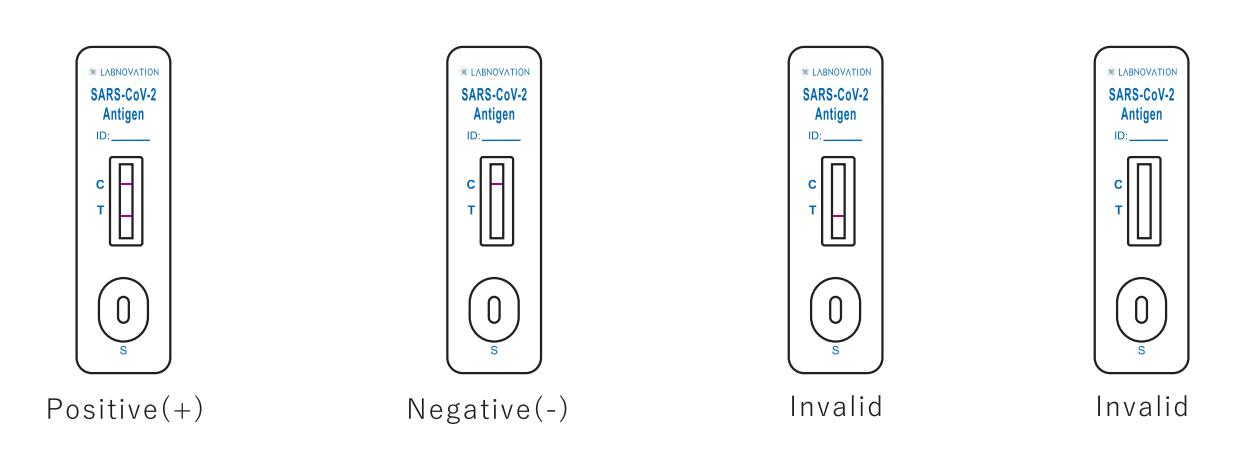
Result Interpretation
Positive: Two colored bands appear on the membrane. One band appears in the control region (C) and another band appears in the test region (T).
Negative: Only one colored band appears, in the control region (C). No apparent colored band appears in the test region (T).
Invalid: If there is no Control line (C) or only a Test line (T) in the result window, the test did not run correctly and the results are not valid.
Virus Sources
| Global high frequency mutation | Alpha / B.1.1.7(U.K.) | Beta I B.1.351(South Africa) |
| Gemma I P.1(Brazil) | Kappa I B.1.617.1(India) | Delta I B.1.617.2(India) |
| C.37,ect | Alpha I B.1.17(U.K.) | B.1.36.16.etc |
| A.2.5,etc | A.23.1 | Alpha I B.1.17(U.K.) |
| B.1.1.33.etc | C.1.1.etc. | Omicron |
Precautions
FAQ
We can send by Express, Air cargo or Ocean cargo.
As we're manufacturer, we make sure to offer you the best quality with competitive price.
We accept many payment term, such as T/T, D/P, D/A etc.
Certificate

|