| Sign In | Join Free | My frbiz.com |
|
| Sign In | Join Free | My frbiz.com |
|
| Categories | Antigen Rapid Test Kit |
|---|---|
| Brand Name: | LABNOVATION |
| Model Number: | LX-401301 |
| Certification: | ISO13485,ISO9001,CE |
| Place of Origin: | China |
| MOQ: | 10000 Pieces |
| Price: | Negotiation |
| Payment Terms: | T/T, D/A, D/P |
| Supply Ability: | 500000/Day |
| Delivery Time: | 5-7 work day |
| Packaging Details: | Carton |
| Specimen: | Nasal Swab / Oropharyngeal swab |
| Test Time: | <15 min |
| Quanlity Guarantee Period: | 24 months |
| Group: | Universal |
| Storage Condition: | 2℃-30℃ |
| Humidity: | ≤60% |
| Company Info. |
| Labnovation Technologies, Inc. |
| View Contact Details |
| Product List |
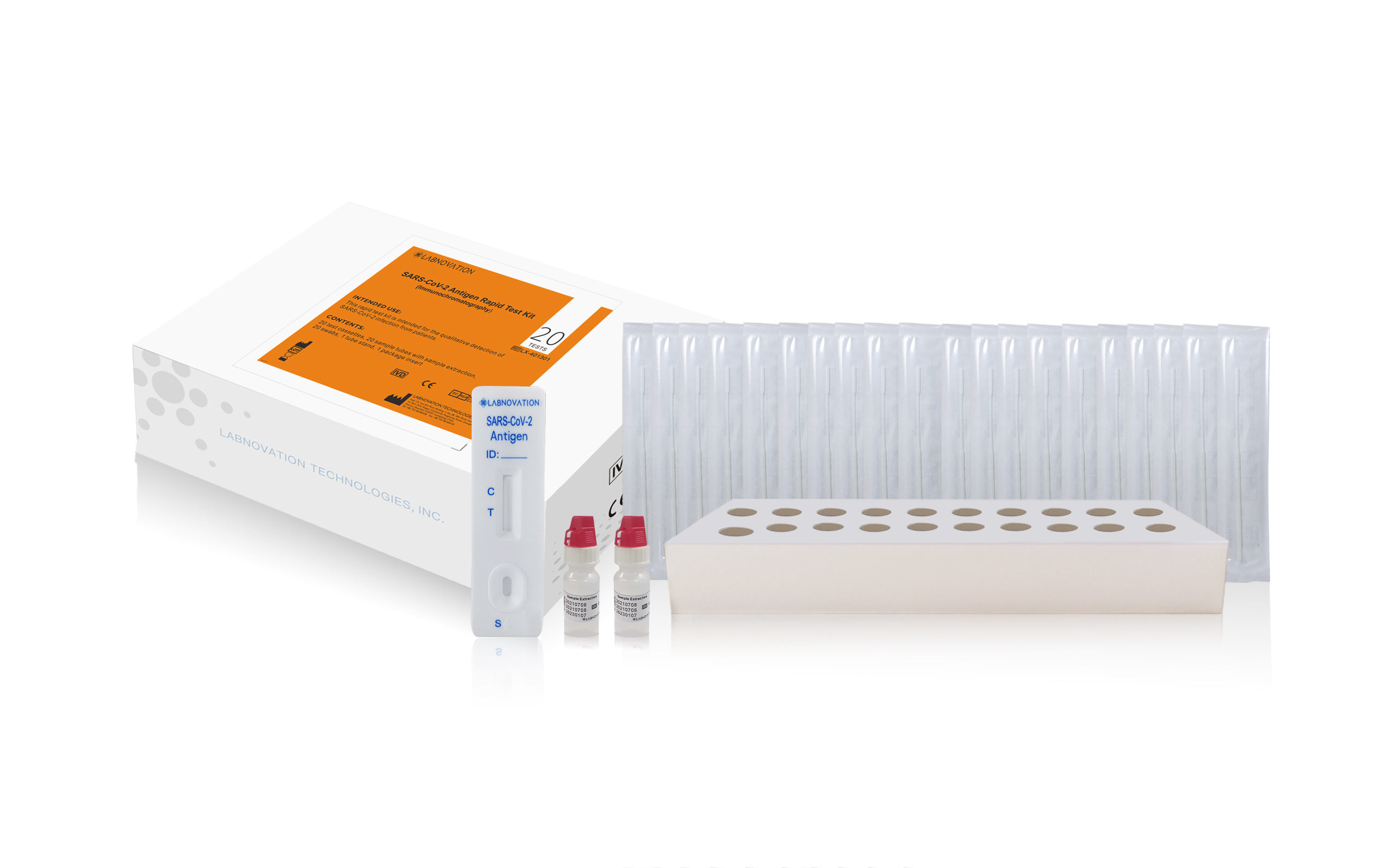
Rapid Antigen Test Kit COVID-19 Antigen Rapid Test 20Test For Professional Testing Use High Quality Antigen Rapid Test Kit
Intend Use
This is used for in vitro qualitative detection of the antigen of novel virus in human nasopharyngeal swabs. It is for professional use only. The test kit in an aid the diagnosis of the patients with suspected SARS-CoV-2 infection. The test kit performance is conjunction with clinical presentataion and results of other laboratory tests. Results from this test kit should not be used as the sole basis for diagnosis.
Product Details
| Item | Value |
| Product Name | SARS-CoV-2 Antigen Rapid Test Kit |
| Model Number | LX-401301 |
| Type | 20 Tests/Box |
| Sensitivity | 98.04%, |
| Specificity | 100% |
| Warranty | 24 Months |
| Quality Certification | CE |
| Safty Standard | ISO13485,MSDS |
| Sample Type | Nasal, Oropharynx Swab |
| Sample volume | 3 Full drops |
| Test Speed | Within 15 minutes |
Product Feature

Application
Main Components

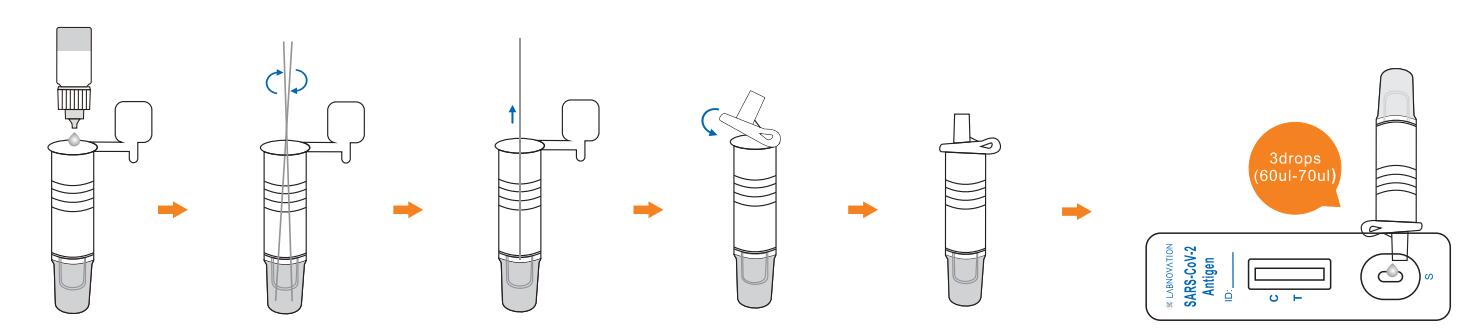
Use Step
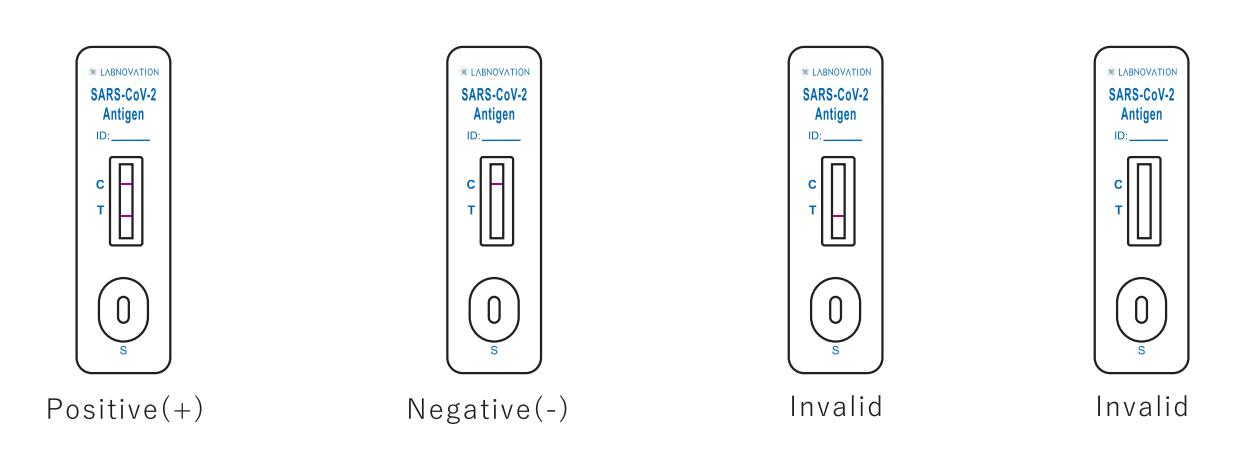
Result Interpretation
POSITIVE: Two (2) distinct colored lines appear. One line should be in the control region (C) and the other line should be in the test region (T).
NEGATIVE: One (1) colored line appears in the control region(C). No apparent colored line appears in the test region (T).
INVALID: No colored lines appear, or control line fails to appear, indicating that the operator error or reagent failure.
Virus Sources
| Global high frequency mutation | Alpha / B.1.1.7(U.K.) | Beta I B.1.351(South Africa) |
| Gemma I P.1(Brazil) | Kappa I B.1.617.1(India) | Delta I B.1.617.2(India) |
| C.37,ect | Alpha I B.1.17(U.K.) | B.1.36.16.etc |
| A.2.5,etc | A.23.1 | Alpha I B.1.17(U.K.) |
| B.1.1.33.etc | C.1.1.etc. |
Certificate
FAQ
You can judge from 3 facts:
Technical data: Such as the accuracy, specificity and sensitivity.
Pouch sealing: Tight enough. If the foil pouch is not sealed well, the humidity in circustance will destroy the reactivity of antibodies labeled on NC membrane. Shelf life will be shorten down.
Background: Good test usually gives clean background after running. If there are red smears in the reading window, it usually caused by bad colloidal gold technology or bad NC membrane. Sometimes, the defect caused false positive result in practice.
We have the MOQ limit, which is 10000 pieces.
CE, ISO9001, ISO13485
After order confirmed, we will arrange your order immediately, and offer you an estimated delivery date.
Business to business account.

|