| Sign In | Join Free | My frbiz.com |
|
| Sign In | Join Free | My frbiz.com |
|
| Categories | Neutralizing Antibody Test Kit |
|---|---|
| Brand Name: | LABNOVATION |
| Model Number: | LX-401701 |
| Certification: | ISO13485, ISO9001, CE |
| Place of Origin: | China |
| MOQ: | 10000/Pieces |
| Price: | US $0.7-1/Piece |
| Payment Terms: | D/A, D/P, T/T |
| Supply Ability: | 500000/Day |
| Delivery Time: | 5-7 work day |
| Packaging Details: | Carton |
| Specimen: | Serum, Plasma, Whole Blood |
| Storage: | 2℃-30℃ |
| Group: | All People |
| Test Speed: | Within 15 minutes |
| Specimen Type: | whole blood, serum, plasma |
| Company Info. |
| Labnovation Technologies, Inc. |
| View Contact Details |
| Product List |
Rapid Test Kit Neutralizing Antibody Detection 20 Tests For Professional Use
Intended Use
The SARS-CoV-2 Neutralizing Antibody Rapid Test Kit is intended use as an aid in identifying individuals with an adaptive immune response to SARS-COV-2, indicating recent or prior infection or assisting in evaluating the effectiveness of the vaccine clinical trials and mass vaccination.
Specifications
| Test Item | Neutralizing Antibody Test Kit |
| Number | LX-401701 |
| Specimen Type | Whole blood, Serum, Plasma |
| Sample Volume | Whole blood 20µL, Serum/Plasma 10µL |
| Package | 20 Test/Box |
| Specificity | 98.00% |
| Sensitivity | 97.67% |
| Accuracy | More than 95% |
| Shelf Life | 18 Months |
| Type | IVD Antibody Rapid Test |
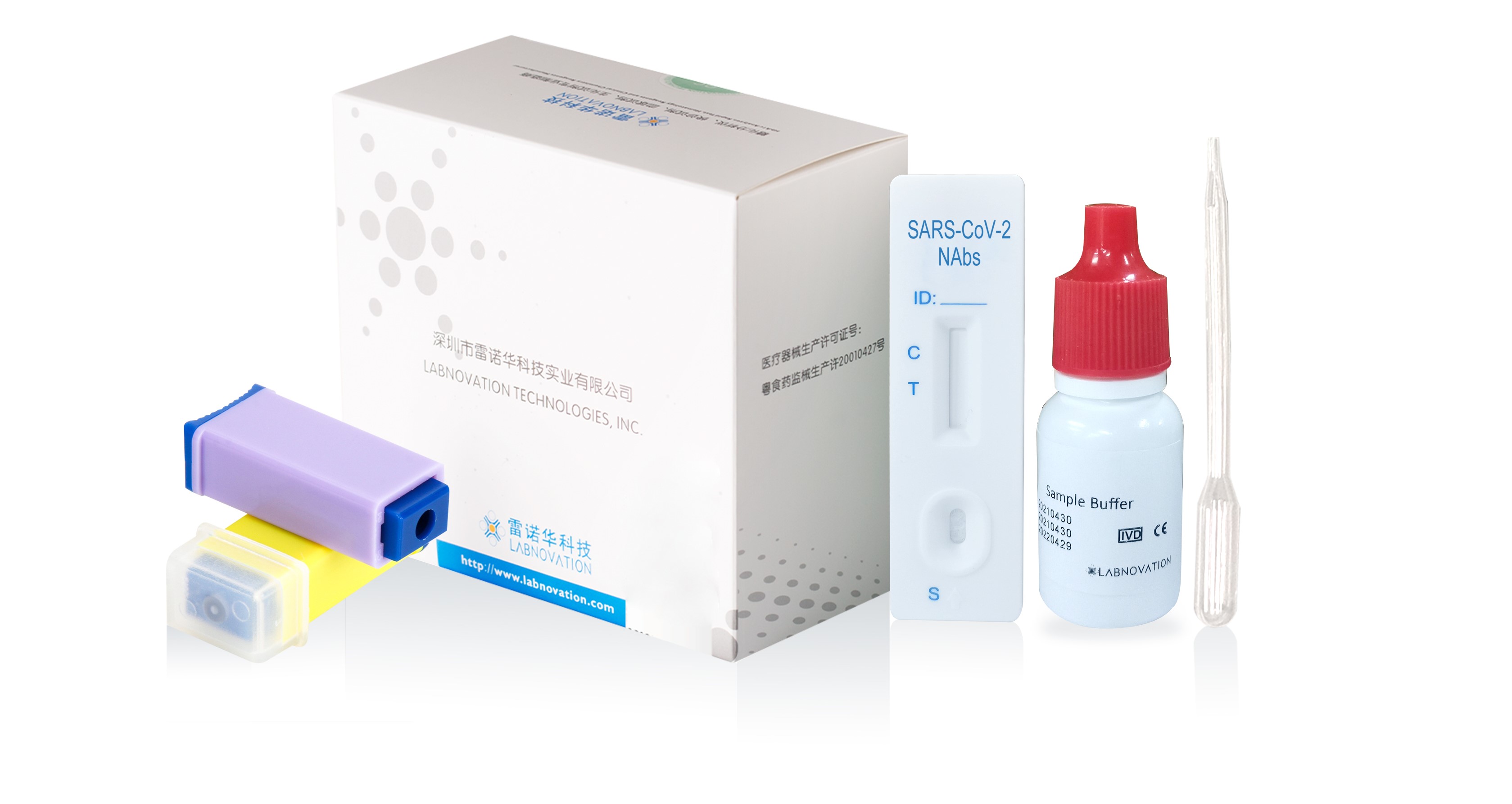
Main Components
Product Feature

Principle
The test employs the sandwich immunoassay to detect the total neutralizing antibodies. The color intensity of the T line is correlated with the level of the neutralizing Antibody in specimen. To determine the level of the neutralizing antibody in specimen, comparing the intensity of test line to the attached colorimetric chart.
Use Step
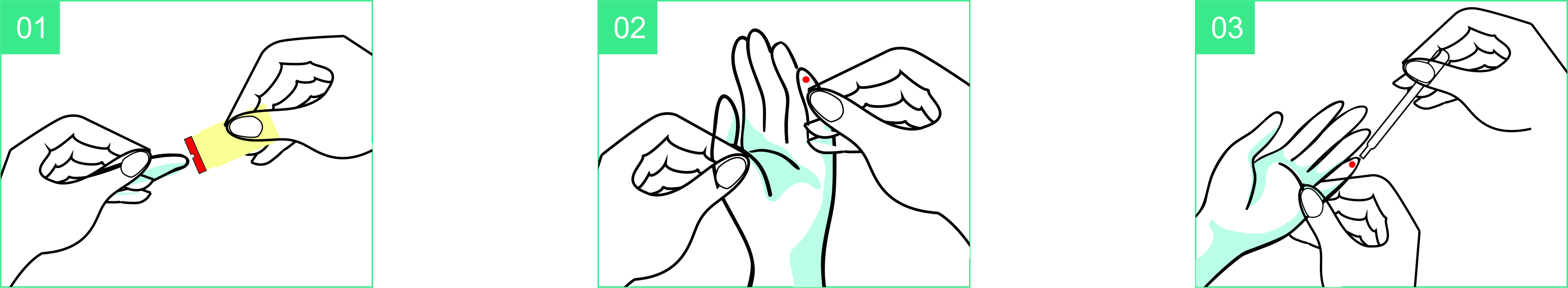
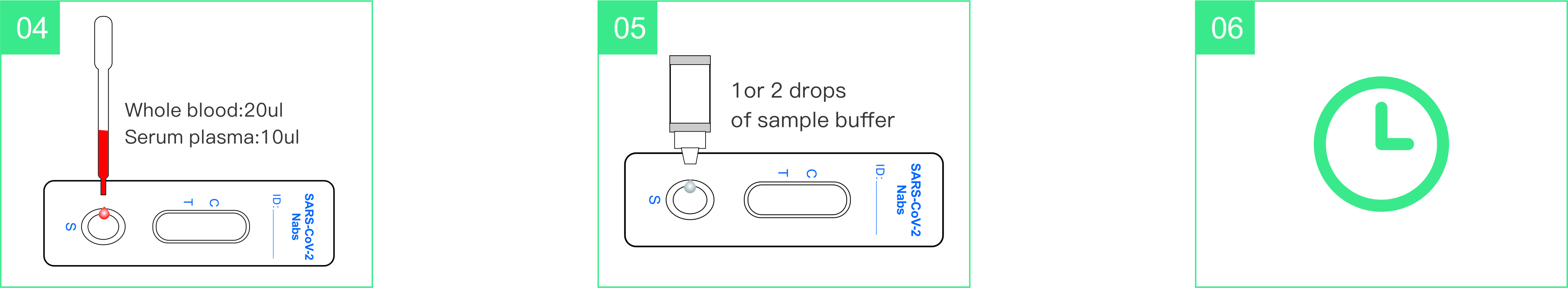
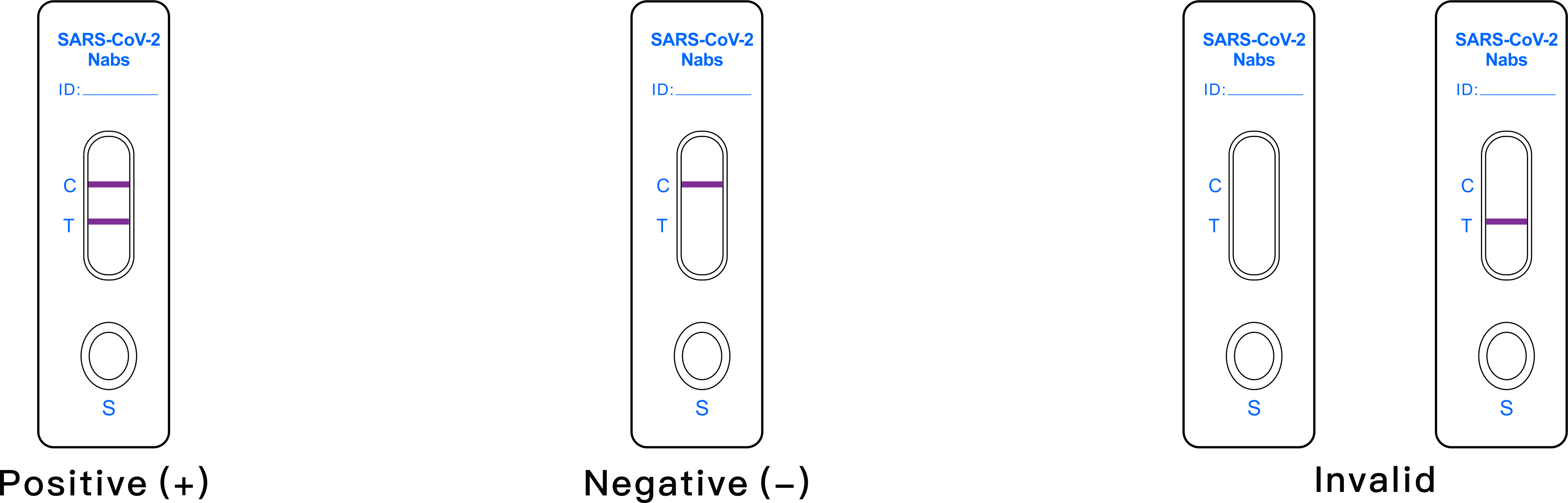
Interpretation of results
Other Information
Application
Evaluation of Vaccination: An aid in theevaluation of individual immune response after vaccination
Diagnosis of infection: An aid in the diagnosis of individuals with suspected SARS-CoV-2 infection in conjunction with other tests and clinical lab.
Epidemiology Research: Immune surveillance of large populations or regions.
Assessment of Convalescent Plasma Therapy: Determine who may quality to donate blood for convalescent plasma therapy for sever COVID-19 patients.
FAQ
We have the MOQ limit,which is 10000 pieces.
After order confirmed,we will arrange your order immediately,and
offer you an
estimated delivery date.
Business to business account.
We choose Air cargo or Ocean cargo.
Manufacturer

|