| Sign In | Join Free | My frbiz.com |
|
| Sign In | Join Free | My frbiz.com |
|
| Categories | Saliva Antigen Test Kit |
|---|---|
| Brand Name: | LABNOVATION |
| Model Number: | LX-401603 |
| Certification: | ISO13485,ISO9001,CE |
| Place of Origin: | China |
| MOQ: | 10000 Pieces |
| Price: | Negotiation |
| Payment Terms: | T/T, D/A, D/P |
| Supply Ability: | 500000/Day |
| Delivery Time: | 5-7 work day |
| Packaging Details: | Carton |
| Test Time: | Within 15 min |
| Storage Condition: | 2℃-30℃ |
| Shelf Life: | 24 Months |
| Application: | Universal |
| Classification: | IVD Test Kit |
| Color: | White |
| Company Info. |
| Labnovation Technologies, Inc. |
| View Contact Details |
| Product List |
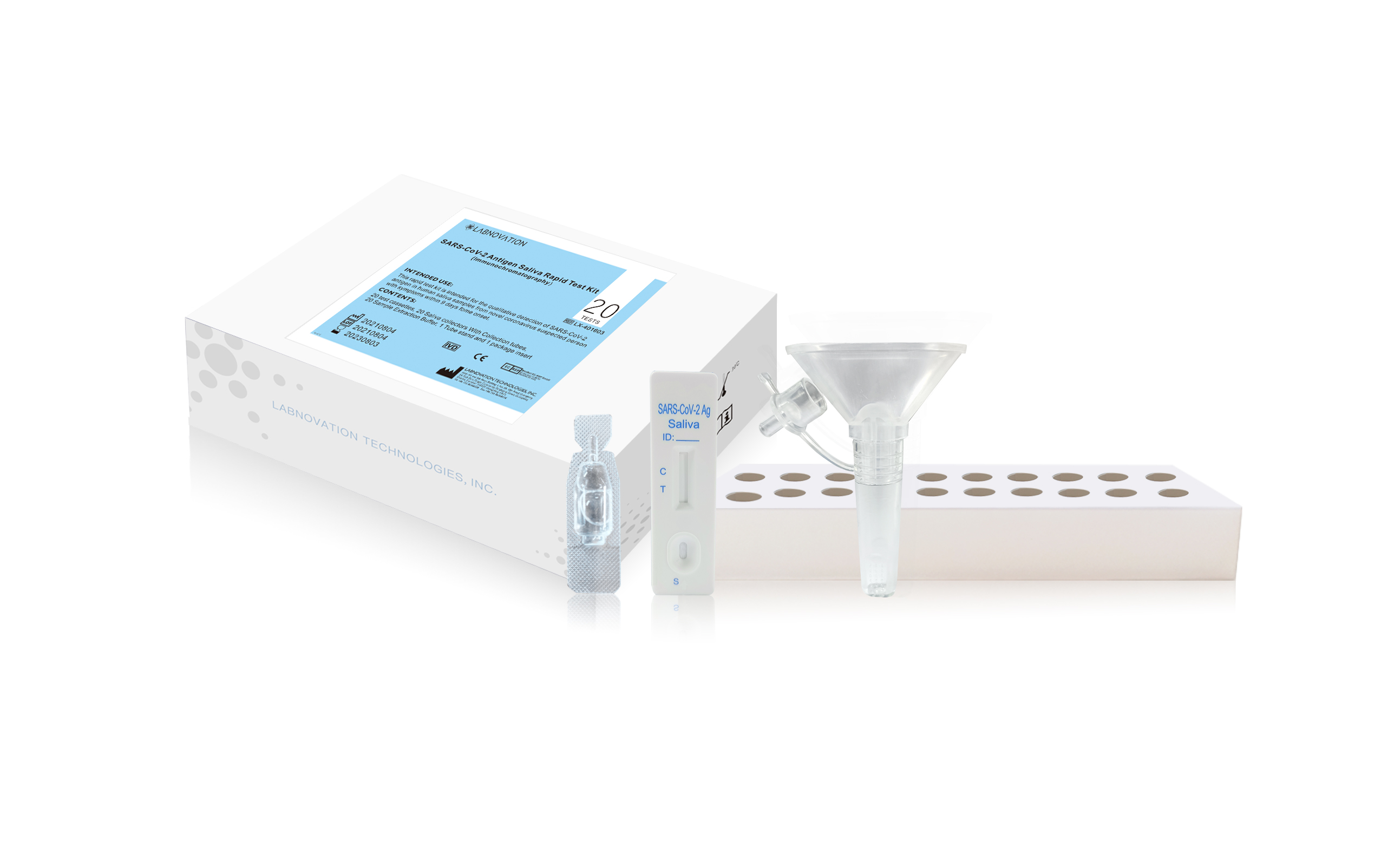
Rapid Test Kit SARS-CoV-2 Antigen Saliva Rapid Test Kit 20 Tests For Professional Use Antigen Rapid Test Kit
Intend Use
This rapid test kit is qualitative detection of SARS-CoV-2 antigen in saliva sample which can be used to detection of SARS-CoV-2 antigen in human saliva samples from novel coronavirus suspected person with symptoms within 9 days from onset. The test result can be used for early triage and rapid management of suspected populations, but it cannot be used as diagnosis basis of SARS-CoV-2 infection. Further nucleic acid detection should be carried out for suspected population whose antigen test result is positive or negative.
Product Details
| Item | Value |
| Model Number | LX-401603 |
| Type | 20 Tests/ Kit |
| Quality Certification | CE, MSDS |
| Safty Standard | ISO13485, ISO9001 |
| Specimen Type | Saliva |
| Sample volume | 3 Full drops |
| specificity | 99.00% |
| sensitivity | 94.29% |
| Total Accuracy | 98.02% |
Product Feature
Test Principle
This kit is an immunochromatography assay. According to the gold immunochromatographic test principle, double antibody sandwich method was used to detect SARS-CoV-2 nucleocapsid antigen in the samples.

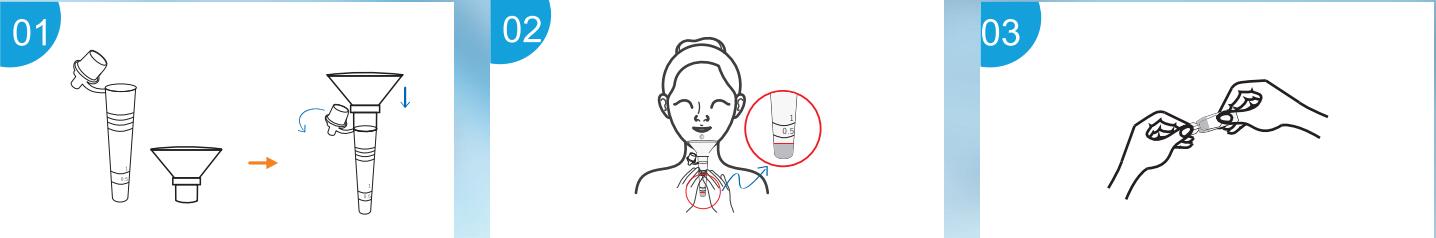
Sample Collection
Use Step
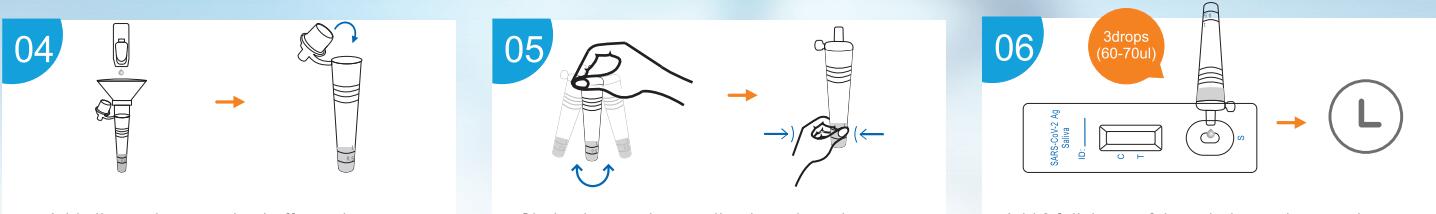
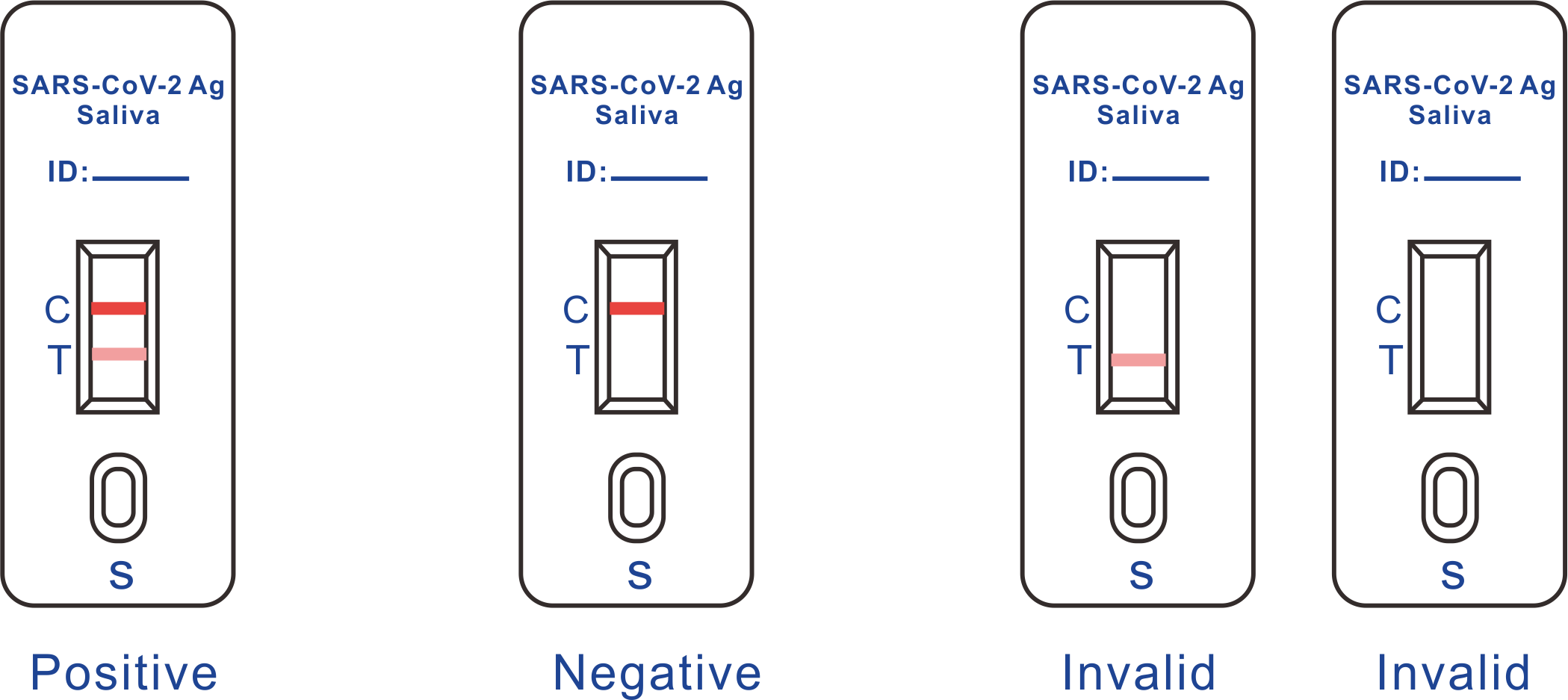
Result Interpretation
POSITIVE: Two (2) distinct colored lines appear. One line should be in the control region (C) and the other line should be in the test region (T).
NEGATIVE: One (1) colored line appears in the control region(C). No apparent colored line appears in the test region (T). The negative result does not indicate the absence of analytes in the sample, it only indicates the level of tested analytes in the sample is less than the minimum detection limit.
INVALID: No colored lines appear, or control line fails to appear, indicating that the operator error or reagent failure. Verify the test procedure and repeat the test with a new testing device.
Virus Sources
| Global high frequency mutation | Alpha / B.1.1.7(U.K.) | Beta I B.1.351(South Africa) |
| Gemma I P.1(Brazil) | Kappa I B.1.617.1(India) | Delta I B.1.617.2(India) |
| C.37,ect | Alpha I B.1.17(U.K.) | B.1.36.16.etc |
| A.2.5,etc | A.23.1 | Alpha I B.1.17(U.K.) |
| B.1.1.33.etc | C.1.1.etc. | Etc. |
Certificate

|