| Sign In | Join Free | My frbiz.com |
|
| Sign In | Join Free | My frbiz.com |
|
| Categories | COVID-19 Test Kit |
|---|---|
| Brand Name: | ZOSBIO |
| Model Number: | 2019-NCoV Ag Rapid Test |
| Certification: | CE BfArm |
| Place of Origin: | China |
| MOQ: | To be negotiated |
| Price: | To be negotiated |
| Supply Ability: | 10000 Kit/Kit per Day |
| Delivery Time: | To be negotiated |
| Packaging Details: | Kit |
| Product Name: | 2019-nCoV Ag Rapid Test (Immunochromatography) |
| Certificate: | CE BfArm |
| Packing Specification: | 1 test/kit, 5 tests/kit, 10 tests/kit, 20 tests/kit, 25 tests/kit, 50 tests/kit, 100 tests/kit. |
| Sample buffer: | Phosphate, sodium azide, etc |
| Composition: | Test card, sample buffer and swab |
| validity period: | It was kept at 2 ℃ to 30 ℃ for 18 months |
| Validity of aluminum foil bag: | An hour |
The Kkit was purchased on the qualitative examination of the
ncov-ag. Nasal exchange specimens were collected
As a new weapon, the 2019 NCOV is the major clinical symptom of β
viral pneumonia is fever, weakness, and cough. Many patients
develop symptoms such as a straight nose, runny nose, sore throat,
or diarrhea. critically ill patients usually develop difficulty
breathing or hypooxemia after a week. Serious people will quickly
develop into acute respiratory difficulty syndrome, septic shock,
my dental metabolic poisoning, and um blood disorders.
Through a side immune examiner, the suspected patient ncovag. was
detected in 2019 Antigens were detected in nasal experiments.
Benign texture and viral antigens are essential to confirm the
clinical association between the patient's medical history and
other diagnostic information. Benign texture along with bacterial
infections or other viruses.
Remen is in non-residential environments (e. g.. G. personal
housing and offices, sports activities, airports, and schools). The
test results of this kit and provide clinical reference.
Comprehensive analysis through clinical symptoms and other
examiners.
The agent is an immunoassay kit based against the antibody coforce. The 2019 ncov monoclonal antibody table was placed on binding pads. In the test project, combined with the label test material, the ag 19 cov monoclonal antibody and ag-ab complex were captured on other 2019 ncov monoclonal antibodies and moved upward through the capillary effect as a sandwich complex. If the 2019 NCO VAG exists in the sample, red tape appears in the T area of the window. Otherwise, the result is negative. The Control Line (C) is used for program control. If the test program runs successfully, always display.
The ruler on the reagent is wiped with a test card, a sample
buffer, and a rag.
Test card: composed of aluminum foil, desiccant, test frost and
plastic card. The test sheet consists of absorbing paper, knitted
rocellulose film, test pads, adhesive pads, and rubber sheets. The
2019 NVab(test line was covered with the T line of the Nitro
cellulose membrane) and the goat-mouse polyclonal AB(mass control
line) with the C line and 2019 NVAB. On the mat.
Sample buffer: phosphate, sodium nitride, etc.
Keep it at 2℃~30℃, and the validity period is tentatively set at 18 months.
The aluminum foil strip is valid for one hour.
Production LOT No.: See: Detailed Tabel.
For more information, please refer to talabel.
(1) Collect nose: wipe the sample, insert the nasal cavity, tip trembling 2.5 centimeters at the nasal edge. Bend # 5 along the nasal mucosa repeatedly with the same rag (see Figure 1)
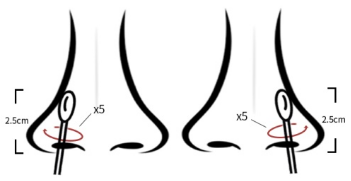
Figure 1 Collection method for nasal swab
(2) Sample treatment: The recovered samples shall be treated with the sample buffer provided in the box as soon as possible (not treated immediately, stored in dry, sterilized and sealed containers) -702 hours. C(to avoid freezing)
Please read the instructions carefully before testing. All the
reagents were determined at room temperature. Room-temperature
test.
(1) Test material treatment technology (see Figure 2)
Insert the sample exchange fluid into the sample buffer and rotate
to about 10 times the inner wall to dissolve the sample as
dissolved in solution as possible.
1. injected the liquid into the tube in front of the inside wall of
the tube and throws it away.
2.. Cover it with a hair dryer.
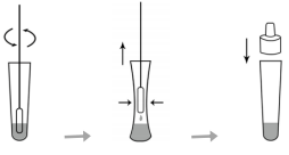
Figure 2 Sample processing
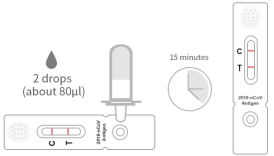
Figure 3 Detection procedure
(2) Examination order (see Figure 3)
The 1. took out the test card.
The 2. two drops plus μ50) places the test material into the test
card for the taymer to stop working.
Read the texture and after 15 minutes at room temperature. Invalid
results after 5 minutes
Test card description (Figure 4)
Invalid ① result: Quality Control Line (C line) is invalid, if no
response line, you need to check again.
② negative result: Quality control line (C) is a red ribbon.
③ positive texture and are all colors of two red bands, sword
lateral line (T) and Quality Control Line (C).
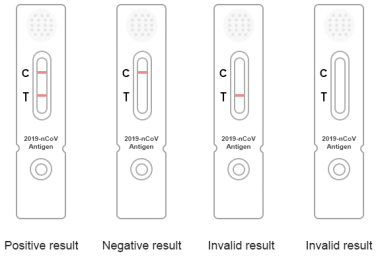
Figure 4 Interpretation of test results
1. This product is only used for in vitro assisted diagnosis by
qualitative inspectors.
2. This product is suitable for the nose shape. Other sample types
are textures and may be negative or invalid.
The 3. test samples have increased significantly. Samples are too
large or too small, and the results may be inaccurate.
4. could not be the sole basis for clinical trials and treatment.
The final diagnosis is made only by a comprehensive evaluation of
all clinical and experimental textures.
1. detection limit: inactivated sars-cov-2 virus with a minimum
detection limit of 6 × 102 TID 50 / ML
2. company reference products for test, and the results must meet
the requirements of the company reference products.
Control the 2.1 Access license rate of the product. The p. 1-p. 15
that the enterprise controls the product is certain.
2.2 Product multiple: the enterprise voice control product n1-n10
is voice.
2.3 Test: The minimum test of control product L1-L3, L1 was
negative and L2, L3 was positive.
2.4 Repeatability: Standard substances J 1 and J 2 were repeatedly
used in the Sword Company 10 times.
3.Cross-reaction: To assess the potential hazards of the NCO-VAG
test project in 2019, the following microbes and BARES, were added
to samples of fixed concentration indicating that the
cross-reaction and interference of various microorganisms and
viruses were absent.
| SN | Microorganisms | Concentration | Cross reaction |
| 1 | Coronavirus (HKU1, OC43, NL63 and 229E) | 1.0×105TCID50/mL | No |
| 2 | H1N1 influenza (new type A H1N1 influenza virus (2009), seasonal H1N1 influenza virus), H3N2, H5N1, H7N9 | 1.0×105TCID50/mL | No |
| 3 | Influenza B (Yamagata strain, Victoria strain) | 2.5×105TCID50/mL | No |
| 4 | Respiratory syncytial virus | 2.8×105TCID50/mL | No |
| 5 | Group A, B, C of rhinovirus | 2.0×105TCID50/mL | No |
| 6 | Type 1, 2, 3, 4, 5, 7, 55 of adenovirus | 2.0×105TCID50/mL | No |
| 7 | Group A, B, C and D of enterovirus | 2.0×105TCID50/mL | No |
| 8 | EB virus | 2.0×105TCID50/mL | No |
| 9 | Measles virus | 2.0×105TCID50/mL | No |
| 10 | Human cytomegalovirus | 2.0×105TCID50/mL | No |
| 11 | Rotavirus | 2.0×105TCID50/mL | No |
| 12 | Norovirus | 2.0×105TCID50/mL | No |
| 13 | Mumps virus | 2.0×105TCID50/mL | No |
| 14 | Varicella-zoster virus | 2.0×105TCID50/mL | No |
| 15 | Mycoplasma pneumoniae | 1.0×106CFU/mL | No |
| 16 | Legionella pneumophila | 1.0×106CFU/mL | No |
| 17 | Haemophilus influenzae | 1.0×106CFU/mL | No |
| 18 | Streptococcus pyogenes (group A) | 1.0×106CFU/mL | No |
| 19 | Streptococcus pneumoniae | 1.0×106CFU/mL | No |
| 20 | Escherichia Coli | 1.0×106CFU/mL | No |
| 21 | Pseudomonas aeruginosa | 1.0×106CFU/mL | No |
| 22 | Neisseria meningitidis | 1.0×106CFU/mL | No |
| 23 | Candida albicans | 1.0×106CFU/mL | No |
| 24 | Staphylococcus aureus | 1.0×106CFU/mL | No |
4. interference: The 2019 NCO-VAG trial was planned to add the following concentrations of drugs to the sample to assess potential hazards. The results showed that all the drugs did not hinder the reagent examination results and.
| Interfering substances | Concentration | Interfering substances | Concentration |
| Mucoprotein | 1mg/mL | Ribavirin | 0.4mg/mL |
| Whole Blood | 1% | Fluticasone | 0.5mg/mL |
| Oxymetazoline | 10mg/mL | Dexamethasone | 5 mg/mL |
| Histamine hydrochloride | 10mg/mL | Triamcinolone acetonide | 5mg /mL |
| Tobramycin | 1mg/mL | Levofloxacin | 0.2 mg/mL |
| Oseltamivir | 1mg/mL | Azithromycin | 0.1 mg/mL |
| Zanamivir | 1mg/mL | Ceftriaxone | 0.4 mg/mL |
| Arbidol | 0.5mg/mL | Meropenem | 0.2 mg/mL |
5. hook effect: within the high concentration range of 1.0. No. ×
106 tcid 50 / ml,19 ncov inactive cultures were not found.
6. clinical extension area was evaluated with 120 positive and 120
negative (RT-PCR side) with heavy water reagents. Textures and are
equal to the following.
| Nasal swab | RT-PCR | Sum | ||
| Positive | Negative | |||
| Positive | 116 | 2 | 118 | |
| Negative | 4 | 118 | 122 | |
| Sum | 120 | 120 | 240 | |
| Sensitivity | 96.67%, (95%CI: 91.74%~98.70%) | |||
| Specificity | 98.33%, (95%CI: 94.13%~99.54%) | |||
1. This product can only be used for in vitro diagnosis.
2. This product is a disposable product and cannot be recycled.
Read the instructions carefully before the 3. operation. The 3.
shall strictly follow the reagent instructions.
The 4. laboratory avoids disinfection of 84, high concentrations of
corrosive gases and dust, such as sodium hypochlorite, acid-alkali,
or aldehydes.
All 5. used samples and reagents should be considered potentially
infectious substances and treated in accordance with local law.
6. uses reagents for the period of validity marked on the external
packaging. Use the test card after removing it from the aluminum
foil pocket.
| Do not re-use | Store at 2℃~30℃ | ||
| Consult instructions for use | In vitro diagnostic medical devic | ||
| Batch code | Use-by date | ||
| Keep dry | Keep away from sunlight | ||
| Authorized representative in the European Community | Manufacturer |
 | ZHONGXIU SCIENCE AND TECHNOLOGY CO.,LTD Dingluan industrial zone ,Changyuan City,453400,P.R.CHINA Tel:+86-371-55016575 Email:zosbio@zosbio.com Web:www.zosbio.com |
SUNGO Europe B.V. Olympisch Stadion 24, 1076DE Amsterdam, Netherlands |





|