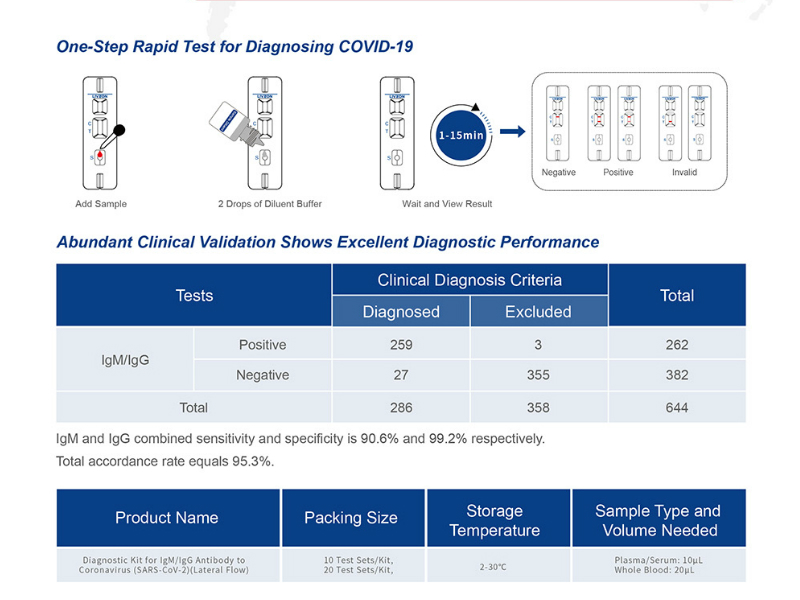
Company Profile
Hebei Pengyuan optoelectrionics Co., Ltd. established in 2006,
adheres to the business philosophy of "innovation, speed and
development", establishes the grand goal of "based on Beijing,
Tianjin and Hebei, facing the whole China and looking at the whole
world", occupies the strategic commanding point of high-tech
industry by relying on independent innovation, and rapidly develops
into a system solution provider of integrating Internet +
Intelligent LED lightning system. Hebei Pengyuan optoelectrionics
Co., Ltd. is a national high-tech enterprise integrating high-end
LED packaging and lighting products research and development,
production, sales and service. And we have comprehensive strategic
cooperation with CRRC group, CR350,CRH380BL,3AG25,CRH-350,CRH-250,
160km/h EMU and other lighting system projects, relying on
scientific and technological innovation, moving to the high end.
In April 2020, Hebei Pengyuan optoelectrionics Co., Ltd. actively
responded to the call of the state, rapidly transformed and
expanded our production into a key epidemic prevention material
production enterprise in Hebei Province, providing effective
guarantee for the global epidemic resistance, and establishing an
industrial chain integrating KN95 particulate respirators research
and development, production, sales. Build 100000 level purification
workshop, introduce 10 production lines of respirators, with a
daily output of up to 2 million. It has full-automatic respirator
production line, computer belt cutting machine, ultrasonic welding
machine, automatic packaging machine, high-speed laser marking
machine and other equipment. Hebei Pengyuan optoelectrionics Co.,
Ltd. select high standard materials, high-quality manufacturing,
high-quality inspection, based on the national GB2626-2006 standard
of China for respirators, combined with other national standards of
different countries, we develop and produce respirators suitable
for different countries and regions. And we have passed CE
certification, ISO9001 quality management system certification,
ISO14001 environmental management system certification, etc.
● Founded in 2006, located in Qinhuangdao of Hebei, National
Hi-tech enterprise
● ISO9001:2015 and ISO14001:2015
● Supplying the world with high quality SUPERNOVA KN95 FFP2 masks
● One million capacity per day, V-trust plant audit
● KN95 China Certification and FFP2 CE Certification
● Charter plane shipping, arrive any destination within 24 hours
Company Display
Our Factory
Certificate