| Sign In | Join Free | My frbiz.com |
|
| Sign In | Join Free | My frbiz.com |
|
| Categories | Herbal medicine powder |
|---|---|
| Brand Name: | complex |
| Model Number: | BGH-313 |
| Certification: | ISO9001/Halal/Kosher |
| Place of Origin: | china |
| MOQ: | 1kg |
| Price: | US$151/kg |
| Payment Terms: | L/C, D/A, D/P, T/T, Western Union, MoneyGram |
| Supply Ability: | 5000000kg per year |
| Delivery Time: | 3 workdays after payment |
| Packaging Details: | 1kg 5kg 25kg two plastic bag inside,aluminum foil bag or drum outside |
| Type: | Herbal Extract |
| Variety: | dipotassium glycyrrhizinate |
| Form: | Powder |
| Part: | rhizome |
| Packaging: | Drum |
| Place of origin: | Shaanxi,China(Mainland) |
| Key word: | dipotassium glycyrrhizinate |
| Color: | white powder with slight yellow cast |
| Water soluble: | good water soluble |
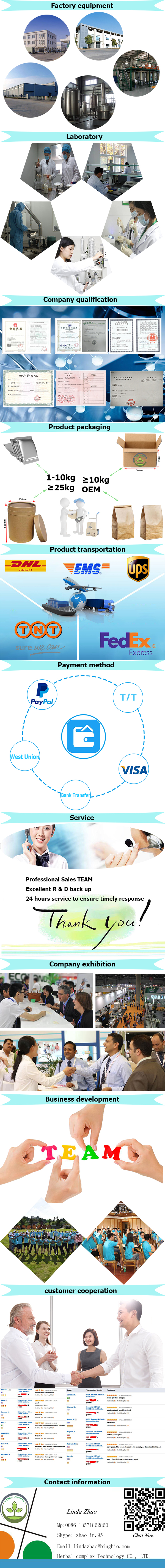
best selling products dipotassium glycyrrhizinate in bulk
Product Details



The potassium salt of glycyrrhizic acid, also known as licorice
root, dipotassium glycyrrhizate is an excellent skin lightener and
brightener.
Derived from the plant Glycyrrhiza glabra, or licorice root, it is
reputed to provide anti-irritant and anti-inflammatory properties
by regulating sebum. In hair care applications, it is claimed to
provide improvements to greasy, oily conditions. Many upscale
cosmetic and body care manufacturers add dipotassium glycyrrhizate
to anti-acne and skin soothing remedies. As a note, glycyrrhizic
acid is used as a sweetener (it is 50 times sweeter than sugar) and
also as a cough suppressant in many cough and cold remedies.
Product Analysis
| Item | Specification | Result | Method |
| BasicProduct Information | |||
| Product name | dipotassium glycyrrhizinate | ||
| Part of the Plant | rhizome | Conform | / |
| Country of Origin | China | Conform | / |
| Organoleptic Data | |||
| Appearance | Fine powder | Conform | NLS-QCS-1008 |
| Color | white powder with slight yellow cast | Conform | GB/T 5492-2008 |
| Odor& Taste | Characteristic | Conform | GB/T 5492-2008 |
| Process Data | |||
| Solvent(s) Used | Water and ethyl alcohol | Conform | / |
| Extract ration | / | Conform | |
| Content | Paeoniflorin≥98% | 98.09 | |
| Drying Method | Spray drying | Conform | / |
| Excipient | Maltodextrin | Conform | / |
| Physical Characteristics | |||
| Particle Size (80 mesh) | 98.0%pass 80mesh | Conform | GB/T 5507-2008 |
| Moisture | <5.0% | 2.3% | GB/T 14769-1993 |
| Ash Content | <4.0% | 1.1% | AOAC 942.05, 18th |
| Solvent Residue | Eur. Pharm | Conform | NLS-QCS-1007 |
| Heavy Metals | |||
| Total Heavy Metals | <10 ppm | Conform | USP <231>, method II |
| Arsenic | <1.0 ppm | Conform | AOAC 986.15, 18th |
| Lead | <1.0 ppm | Conform | AOAC 986.15, 18th |
| Mercury | <0.5 ppm | Conform | AOAC 971.21, 18th |
| Pesticide Residue | |||
| 666 | <0.2ppm | Conform | GB/T5009.19-1996 |
| DDT | <0.2ppm | Conform | GB/T5009.19-1996 |
| Microbiology | |||
| Total Plate Count | <1,000cfu/g | 16cfu/g | AOAC 990.12, 18th |
| Total Yeast & Mold | <100cfu/g | 4cfu/g | FDA (BAM) Chapter 18, 8th Ed. |
| E. Coli | Negative | Negative | AOAC 997.11, 18th |
| Salmonella | Negative | Negative | FDA (BAM) Chapter 5, 8th Ed. |

|